Who Is Exempt From Fordham’s Vaccine Requirement?
The ins and outs of Fordham’s vaccine exemption policy for those with at-risk medical conditions or religious obligations
Students wishing to receive a medical exemption from Fordham must apply for a waiver demonstrating their medical need.
July 5, 2021
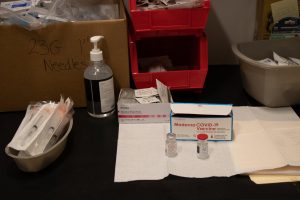
After a year and a half of online classes, Fordham University will finally return to in-person classes in the fall 2021 semester. After Sept. 21, 2021, in order to return to Fordham’s campus, one must have received a World Health Organization-approved vaccine against the SARS CoV-2 coronavirus.
Health experts universally agree that the current vaccines being distributed worldwide are safe and effective against contracting COVID-19. Despite their effectiveness, not everyone is capable of or planning to receive the vaccine. Fordham makes two exceptions for those who wish to return to campus but will not be vaccinated: medical exemptions and religious exemptions.
While religious exemptions — which are granted by Fordham using signed statements and potentially require supporting documents — are based on religious beliefs toward vaccines, those who are medically exempt could suffer physiological harm should they receive the vaccine.
Fordham’s Exemption Guidelines
To attain an exemption and apply for a vaccine waiver, one must submit an exemption form with documents containing well-justified medical reasons or religious objections attached.
Students or faculty who wish to qualify as medically exempt must present a written statement by a certified medical practitioner.
Students with approved vaccination waivers must obtain frequent COVID-19 tests for full campus access. These students will also have more restrictions on campus compared to vaccinated students. Moreover, students without an approved vaccination waiver will not be allowed on campus. Fordham has required all healthy community members to be vaccinated in order to return to campus and attend in-person events and classes.
Who exactly constitutes as medically exempt depends on either temporary or permanent factors of the individual in question. Either way, students or faculty who wish to qualify as medically exempt must present a written statement by a certified medical practitioner affirming that “the student has a health condition which is a valid contraindication to receiving a specific vaccine.”
Fordham is following the Centers for Disease Control and Prevention (CDC)’s guidelines to identify increased risk groups and their exemption from the COVID-19 vaccine. These groups include those with autoimmune conditions like Guillain-Barre syndrome, multiple sclerosis and Bell’s Palsy. People with autoimmune diseases may fail to develop adequate immunity against the virus due to the medication they take, such as methotrexate and rituximab, which initiate cell death and may “dampen the immune system to stop the body from attacking itself.”
Anaphylaxis to vaccine and vaccine components is also a common reason for exemption.
What Is Anaphylaxis?
According to the CDC’s Morbidity and Mortality Weekly Report, anaphylaxis is a life-threatening allergic reaction that occurs rarely after vaccination. After the analysis of the Vaccine Adverse Event Reporting System, it was shown that 21 cases of anaphylaxis were reported after the administration of the 1,893,360 first doses of the Pfizer-BioNTech vaccine. A total of 71% of these adverse cases reacted within 15 minutes of vaccination. All vaccine centers must adhere to CDC guidelines to have necessary supplies for post-vaccination observation periods such as intramuscular injections of epinephrine for suspected allergic reactions.
The rate of anaphylaxis from the Pfizer-BioNTech vaccine is 11.1 people per 1 million doses.
Common clinical symptoms of anaphylaxis include urticarial, diffuse erythematous rash, angioedema, respiratory and airway obstruction symptoms, and nausea. According to a study conducted by Tom T. Shimabukuro, Ph.D. of and director of the Vaccine Distribution and Implementation Task Force at the CDC, 92% of anaphylaxis cases received epinephrine and were treated in health care settings such as emergency departments or intensive care units for endotracheal intubation.
Possible Precursors
The rate of anaphylaxis from the Pfizer-BioNTech vaccine is 11.1 people per 1 million doses. According to the American Academy of Pediatrics, the median age for anaphylaxis is 40 years and 90% were female.
The CDC states that people should not receive the mRNA vaccines if they have a severe allergic reaction whether immediate or to any severity to a previous dose of a mRNA COVID-19 vaccine. These patients are allergic to polyethylene glycol or polylobate. Polyethylene glycol is a polyether molecule that is derived from petroleum for industrial manufacturing and medicine.
People with allergic reactions to other injectable therapies can receive the vaccine but should be informed about the risks and be observed for 30 minutes after the vaccination.